ONFI® (clobazam) is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
ONFI® (clobazam) is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.

Physicians who decide that ONFI is clinically appropriate for their LGS patients should write “DISPENSE AS WRITTEN (DAW)” on the prescription or follow their state’s instructions for indicating branded versus generic products to ensure that patients receive ONFI as intended


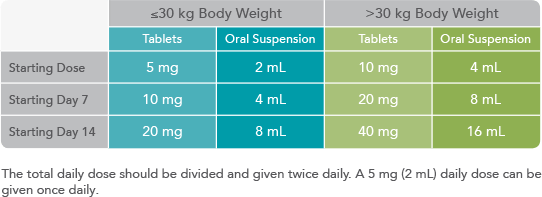
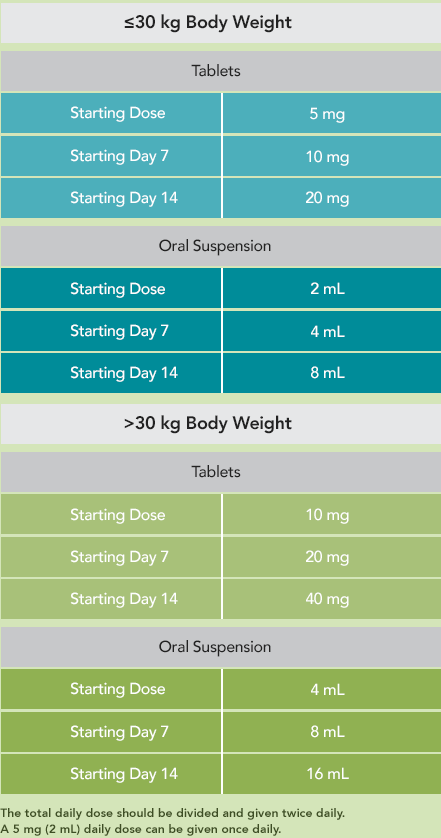
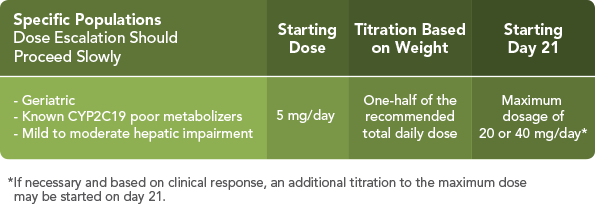
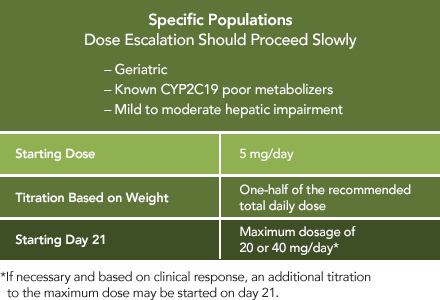
The following AEDs did not significantly alter the pharmacokinetics of ONFI or its active metabolite1:
Cost-savings information, education, and resources to help your patients
Learn about the safety profile and results from our clinical data
Get materials, patient handouts, and other helpful information for your practice
Indications and Usage
ONFI (clobazam) CIV is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
Important Safety Information
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS See full Prescribing Information for complete boxed warning. Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death.
The use of benzodiazepines, including ONFI, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death.
Abrupt discontinuation or rapid dosage reduction of ONFI after continued use may precipitate acute withdrawal reactions, which can be life-threatening.
|
Indications and Usage
ONFI (clobazam) CIV is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
Important Safety Information
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS See full Prescribing Information for complete boxed warning. Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death.
The use of benzodiazepines, including ONFI, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death.
Abrupt discontinuation or rapid dosage reduction of ONFI after continued use may precipitate acute withdrawal reactions, which can be life-threatening.
|
Contraindication: Hypersensitivity
ONFI is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients. Hypersensitivity reactions have included serious dermatological reactions.
WARNING: Risks from Concomitant Use with Opioids
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe ONFI concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use. Advise both patients and caregivers about the risks of respiratory depression and sedation when ONFI is used with opioids.
WARNING: Abuse, Misuse, and Addiction
Abuse and misuse of benzodiazepines often (but not always) involves the use of doses greater than the maximum recommended dosage and commonly involves concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death.
Use of ONFI, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of ONFI along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of Central Nervous System (CNS) depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
WARNING: Dependence and Withdrawal Reactions
Patients at an increased risk of withdrawal reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
The continued use of benzodiazepines, including ONFI, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of ONFI after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures).
In some cases, benzodiazepine users have developed protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months.
Potentiation of Sedation from Concomitant Use with CNS Depressants
ONFI has a CNS depressant effect. Caution patients or their caregivers against simultaneous use with other CNS depressant drugs or alcohol, and that the effects of other CNS depressant drugs or alcohol may be potentiated.
Somnolence or Sedation
ONFI causes somnolence and sedation. In clinical trials, somnolence or sedation was reported at all effective doses and was dose-related. In general, somnolence and sedation begin within the first month of treatment and may diminish with continued treatment. Monitor patients for somnolence and sedation, particularly with concomitant use of other CNS depressants. Caution patients against engaging in hazardous activities that require mental alertness, such as operating dangerous machinery or motor vehicles, until the effect of ONFI is known.
Serious Dermatological Reactions
Serious skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with ONFI in both children and adults during the post-marketing period. Discontinue ONFI at the first sign of rash, unless the rash is clearly not drug-related.
Drug Reaction with Eosinophilia and Systemic Symptoms
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, has been reported in patients taking antiepileptic drugs, including ONFI. DRESS typically, although not exclusively, presents with fever, rash and/or lymphadenopathy, in association with other organ system involvement. Eosinophilia is often present. If such signs or symptoms are present, then the patient should be evaluated immediately. ONFI should be discontinued immediately and not restarted unless an alternative etiology for the signs or symptoms can be established.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including ONFI, increase the risk of suicidal thoughts or behavior in patients. Inform patients, their caregivers, and families of the risk and advise them to monitor and report any emergence or worsening of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. If these symptoms occur, consider whether the occurrence may be related to the AED or illness, because epilepsy itself can increase these risks.
Neonatal Sedation and Withdrawal Syndrome
Use of ONFI late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate. Monitor neonates exposed to ONFI during pregnancy or labor for signs of sedation and monitor neonates exposed to ONFI during pregnancy for signs of withdrawal.
Pregnancy, Registry and Nursing Mothers
Overdosage Management
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). The risk of withdrawal seizures with flumazenil may be increased in patients with epilepsy. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
Adverse Reactions
The most commonly observed adverse reactions reported in an LGS randomized, double-blind, placebo-controlled, parallel group clinical trial of patients who received clobazam as adjunctive therapy (≥10% in any treatment group and at least 5% greater than placebo, respectively) were somnolence or sedation (32% vs. 15%), somnolence (25% vs. 12%), pyrexia (17% vs. 3%), lethargy (15% vs. 5%), aggression (14% vs. 5%), drooling (14% vs. 3%), irritability (11% vs. 5%), ataxia (10% vs. 3%), and constipation (10% vs. 0%).
For more information, please see the full Prescribing Information, including Boxed Warning, Medication Guide, and Instructions for Use, or visit ONFIHCP.com.